The fact that the electrons and other atomic and sub-atomic particles undergo phenomena of interference and diffraction imposed to the physicist conceptual revolutions maybe greater than those caused by the Theory of Relativity.
To fully understand this topic we must remember, at least in its fundamental aspects, what is the phenomenon of the diffraction.
In the daily experience we can observe that when a white light beam hits an opaque diaphragm in which there is an hole, we see on a screen, placed behind the diaphragm with respect to the light source, the luminous image of the same hole. If the source is much far from the diaphragm, the image appears of the same dimensions of the hole and, to a superficial look, it seems sharp and clear.
But if instead of the white light we use a monochromatic light and the hole is very small, the luminous image on the screen appears of greater dimensions than the hole, encircled by rings of decreasing brightness and separated by dark rings.

This is a diffraction figure and we can explicate it only admitting undulatory nature to the light.
In fact if we assume that the diaphragm is hit by plane wave wavefronts (for the distance of the source), for the principle of Huygens, every point of the hole is a source of spherical waves that radiate outward from that point with equal speed and hit a point P of the screen after different times of 'fly' and therefore with different phases. If the difference between the distances of the point P from two points C and A of the hole, the first one in the center, the second one on the edge, is half wavelength λ, the elementary waves interfere negatively producing a dark point. The point P marks therefore the position of the first dark ring and consequently OP is the radius of the luminous central circle.
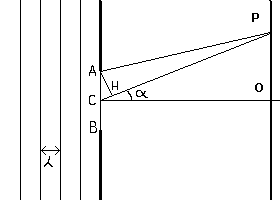
If we say d the diameter AB of the hole, for great distances CO, the difference between beams AP and CP is given from segment CH. The angle CAH is equal to the angle PCO. We shall have therefore
![]()
OP is the radius of the central luminous circle if
![]()
that is
![]()
This relation shows that the sine of α, and therefore also α, increases when d decreases.
If the diaphragm is irradiated with a beam of electrons of speed v perpendicular to it, we obtain a figure of diffraction analogous to that one obtained with the monochromatic light and therefore the relation found for the light is valid also for the electrons.
Multiplying by the momentum p both the terms of this equality we have
![]()
and for the relation of De Broglie
![]()
Every electron that hits the screen is passed though the hole, but we cannot known in which position. Using a system of reference with origin in C and axis of the abscissas directed from C to A, the diameter of the hole is the uncertainty Δx on the position of the electron in the passage.
The momentum p=mv of electrons before the diaphragm is perpendicular to the diaphragm, therefore, the component of the momentum px before the diaphragm is null.
But the electrons hit the screen with a direction that can go from -α to α, so, after the diaphragm, their component px can vary from -psinα to +psinα. psinα measures the uncertainty Δpx on the component x of the momentum. Therefore it results
![]()
The uncertainties on the variables x and px are inversely proportional and their product is of the order of the Plank's constant.
This relation between the uncertainties of two conjugate variables is an example of the uncertainty principle enunciated by Heisenberg, a basis in the foundation of the Quantum Mechanics. This principle formalizes theoretically that the experiences show that the subatomic particles have undulatory behaviour.
The uncertainty principle contrasts the assumption, basic in the Newtonian mechanics, that it is possible to know, at the same time, the position and the speed of a particle. If, for example, we would know with infinite precision the position x of an electron making it to pass through a hole of infinitesimal diameter, we shall obtain an image of the hole with an infinite radius, that is we shall lose any information on the component px of its momentum and therefore of its speed.
While in Classic Mechanics the state of a particle at a given time is perfectly knowable and determines all the states of the past and of the future of the particle, in Quantum Mechanics the pairs of antagonistic variable, like the position and the momentum, are mutually conditioned, making impossible sure forecasts of the future states.
On these states Quantum Mechanics permits only probabilistic previsions.
The uncertainty principle denies the possibility to speak about trajectories and denies the basis of the determinism of the Classic Mechanics, maintained also in the Theory of Special Relativity.