
|

|

|
Liquids which touch pipe's edges rise against these above of level which the liquid reaches far from the edges (concave meniscus) and when one have some communicating vessels, is a capillary (of diameter lower then 1 mm), the liquid rises in this last one until to reach a level higher then that of vessels with the largest area(capillar ascension), due the cohesion forces that are the forces which attract molecules' liquid to that of capillar.

|
Liquids which don'touch the pipe's edges beget opposit phenomenons, due adherence forces which repel molecules' liquid from that of the stain: their meniscus is convex and put in communicating vessels beget capillar depression in the vessel where the area is the lowest. The difference of level h between the capillar ascension (or between the capillar depression) and the normal liquid's level is given by Jurin law:
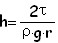
Where τ is a constant which depends from liquid's surface tension, r is the capillar's radius, ρ the density of the liquid and g is the gravity constant; h measure is very delicate because it needs small glass' or liquid's contamination to alter that value.
Capillarity phenomenons are produced by moleculer actions between stain's and the liquid, and they have a big importance for the determination of surface tension and so of liquid's molecular weight considered.
meniscus*:a characteristic curve shape the surface of the liquid try to put on due to the surface tension.