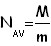|
We first compute the area of the oleic acid stain by performing the half-sum of the 2 values (number of squares) obtained as explained above. As absolute uncertainty of this area we use the half-difference between the 2 counts of squares. In this case having computed the area, and being the volume of oleic acid known, it is easy to find the height of the stain which under the hypothesis of mono-molecular layer, is the same as the linear dimension of a molecule:
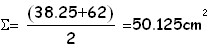
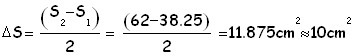
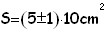
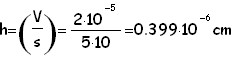
Where V is the volum of an oleic acid drop and not of an oleic acid molecule
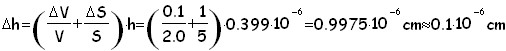
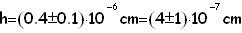
The formula which provide us with the height valle is:in order to proceed we are now forced to we hypotize which the form of a molecule is, (for exemple: a cube,a sphere, etc.) here we'll try 5 different geometrical solid for the oleic acid molecule.
After calculated the oleic acid's molecule's volumes,which depends from its shape, we have to calculate the molecule's mass, because it needs as divisor in the ratio between an oleic acid's mole and its mass.
To calculate the we use this equation:

Where Vm is the volum of the oleic acid's stain, ρ is its density which values 0.9 g/m 3.
When we got the measure of the mass we have to calculate its error:
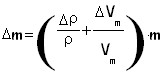
Then we relate an oleic acid's mole with the molecule's mass. From this ratio we deduce how many particels there are in a mole of oleic acid's.